FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Indian pharmacist

FDA Approval of Stem Cell Therapy

FDA Approves First CRISPR/Cas9 Gene-Editing Therapy

Reprogramming stem cells in regenerative medicine - Mao - 2022

Stem Cell Transplantation, Autologous Stem Cell Transplantation
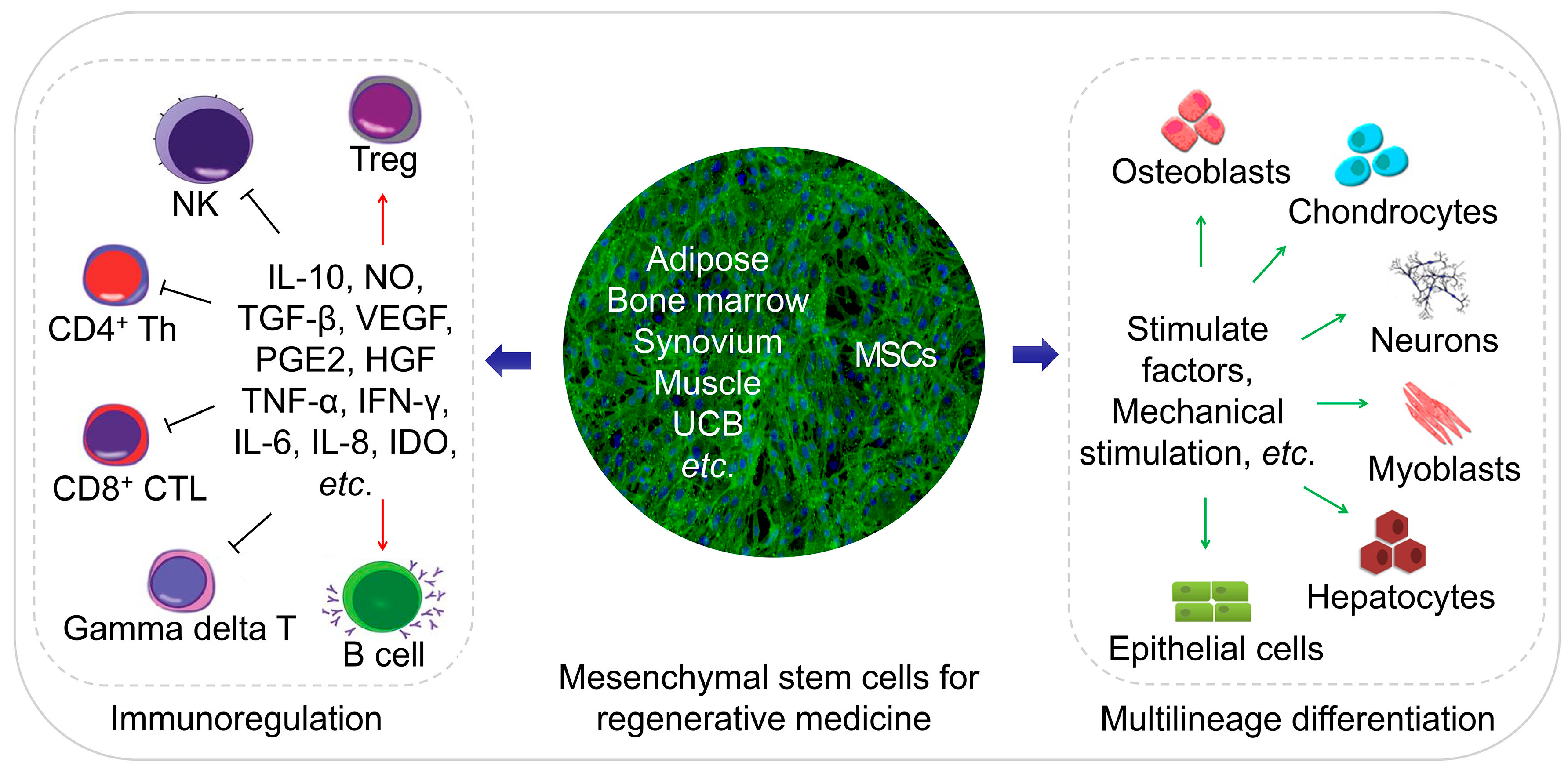
Cells, Free Full-Text
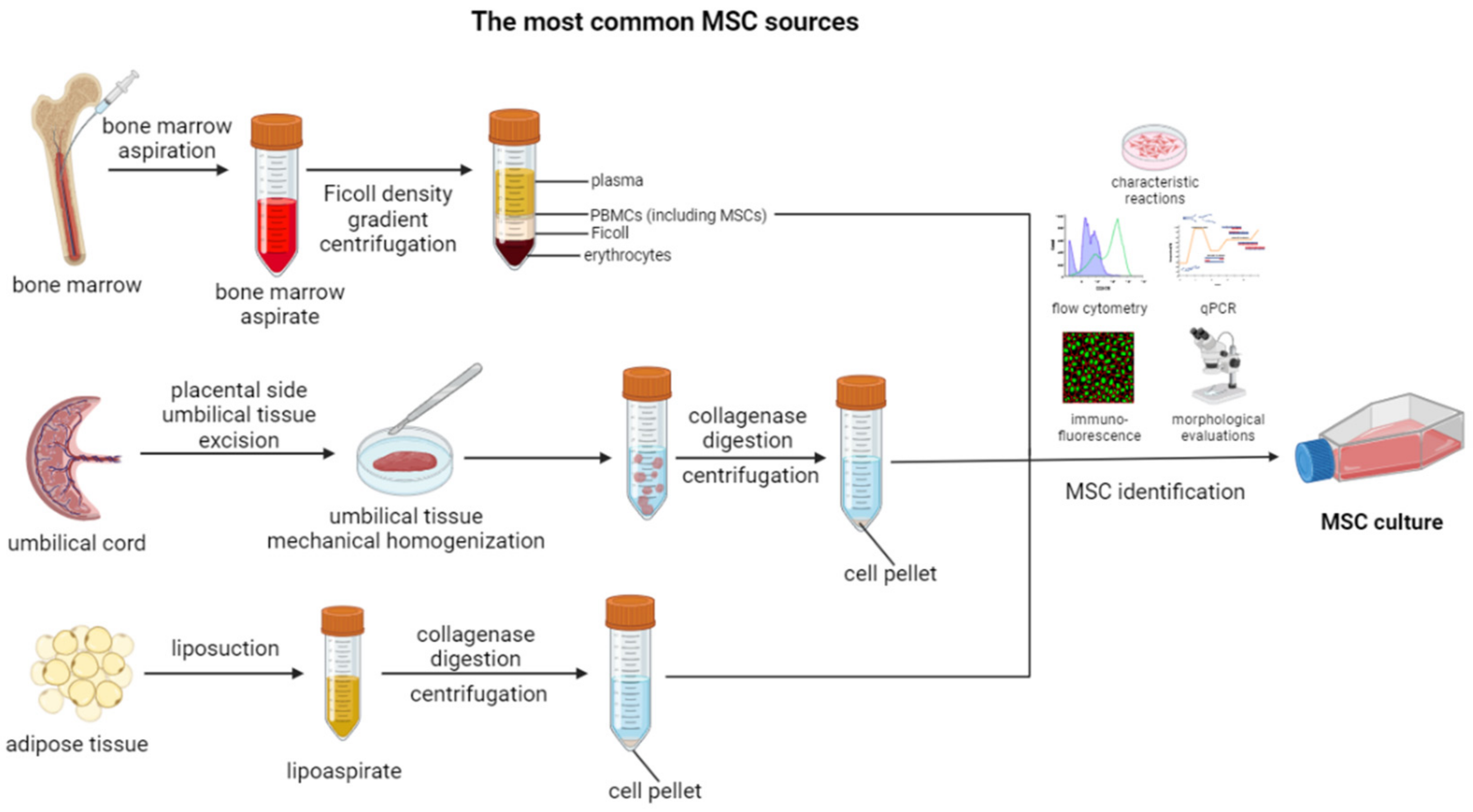
Cells, Free Full-Text
FDA Approves Eflapegrastim-xnst (ROLVEDON) for Chemotherapy

FDA approves cell therapy for patients with blood cancers to

A Judge Rules Against One Stem-Cell Clinic. There Are Hundreds of
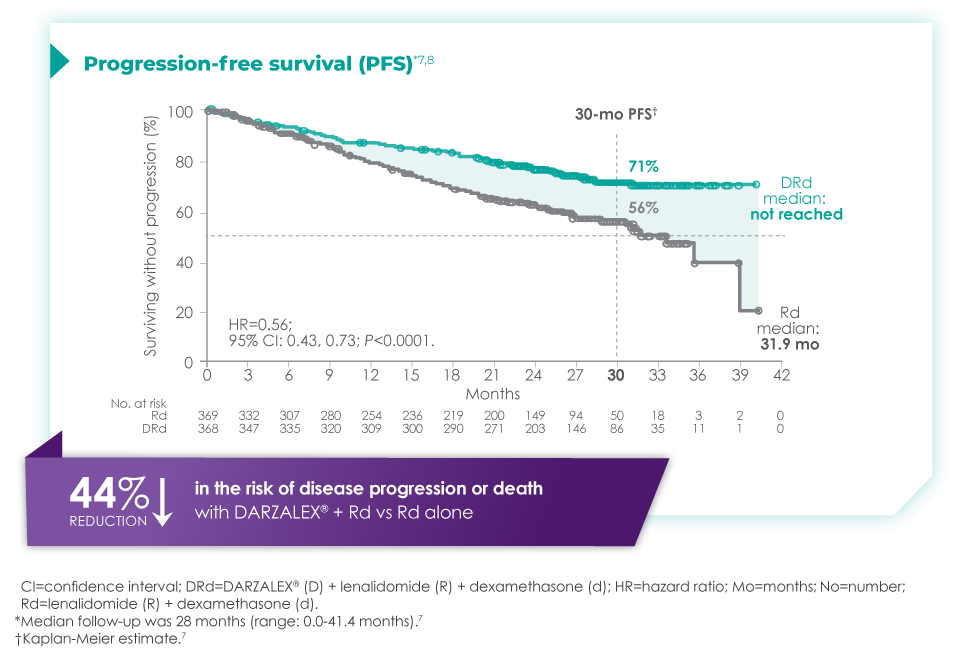
One Expert's Approach in Transplant-Ineligible, Newly Diagnosed

FDA Approves 2 New Bispecifics for Relapsed or Refractory Multiple

Risky Stem-Cell Treatments Come Under F.D.A. Scrutiny — Again
T-cell and natural killer cell therapies for hematologic
de
por adulto (o preço varia de acordo com o tamanho do grupo)







