FDA vaccine advisers 'disappointed' and 'angry' that early data about new Covid-19 booster shot wasn't presented for review last year
Por um escritor misterioso
Descrição
Some vaccine advisers to the federal government say they’re “disappointed” and “angry” that government scientists and the pharmaceutical company Moderna didn’t present a set of infection data on the company’s new Covid-19 booster during meetings last year when the advisers discussed whether the shot should be authorized and made available to the public.
FDA Advisors Call for an End to Never-Ending Boosters, Look Ahead
5 new COVID-related deaths, 1,343 new infections recorded in Hawaii, DOH reports, COVID-19

New covid vaccine booster could be available this week after FDA approval - The Washington Post

News - Page 387 of 1859 - NBC2 News
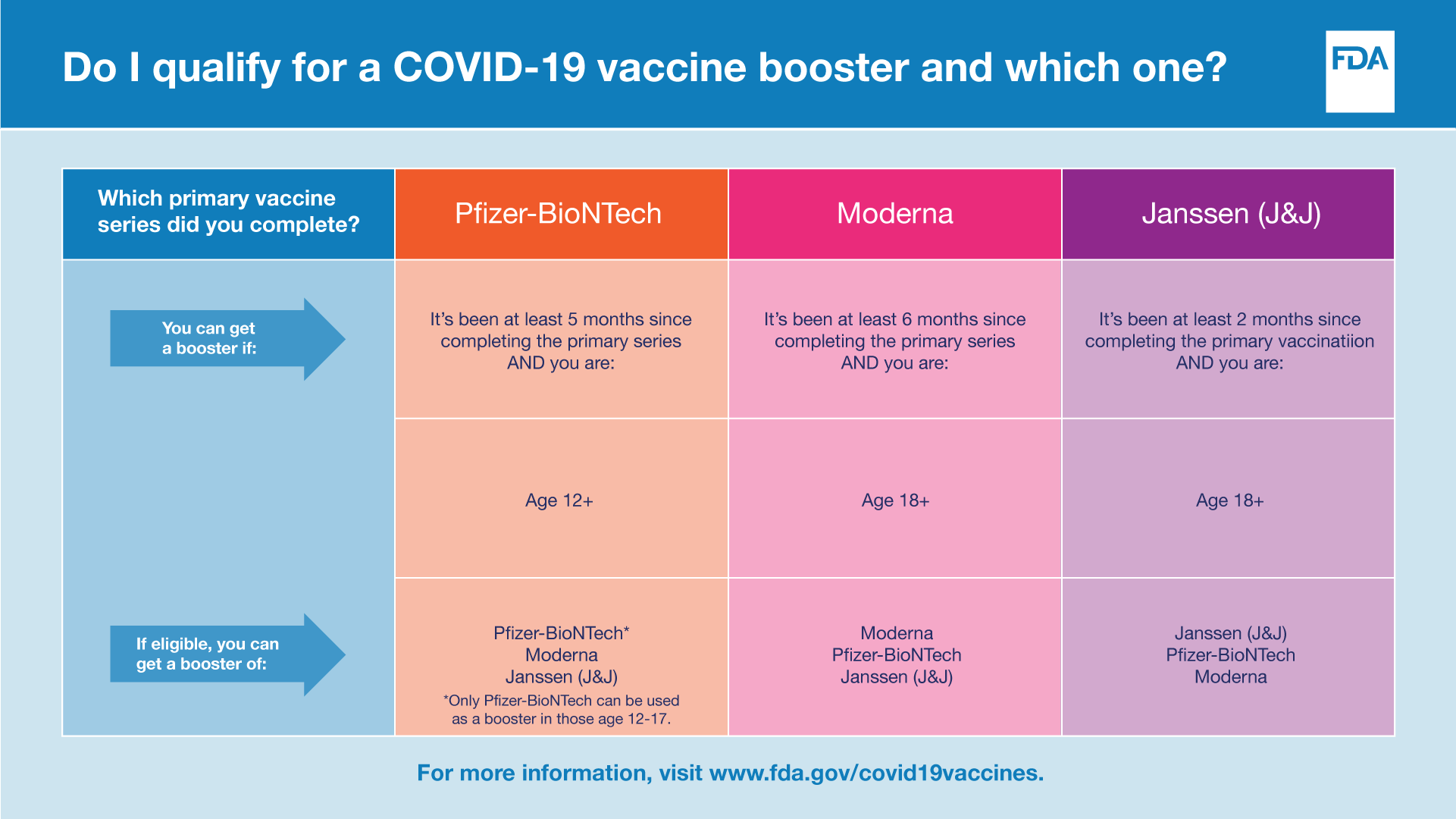
Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine

Watch Bloomberg 'Markets Uproar' Full Show (09/03/2020) - Bloomberg

FDA signs off on new COVID-19 vaccines - updated shots not being called boosters

Jan 11th, 2023 - FDA Did Not Share Booster Data, 400% Price Increase, Michelle Zimmerman; More News

FDA advisory panel rejects widespread Pfizer vaccine booster shots
US FDA considers approving second Covid-19 booster shot – report, Coronavirus
FDA staff say Pfizer COVID-19 boosters may not be needed, but do improve immunity
Covid boosters: FDA panel recommends new XBB shots for the fall

MedWatch
COVID-19 vaccine – One America News Network
de
por adulto (o preço varia de acordo com o tamanho do grupo)







