GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Descrição
GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.

SOLVED: The molar masses and empirical formulas of several compounds containing carbon and nitrogen are listed here. Find the molecular formula of each compound. (a) 163.26 g / mol, C11H17N (b) 186.24

GHGH Formula - C14H26O11 - Over 100 million chemical compounds
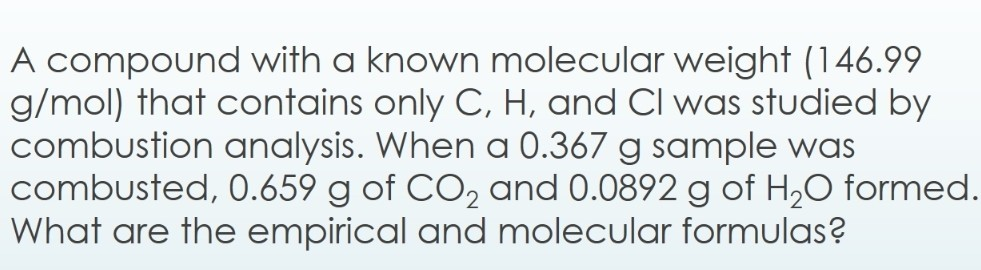
Solved A compound with a known molecular weight (146.99

4-Heptanone SDF/Mol File - C7H14O - Over 100 million chemical compounds

Molecular Formula Percent Composition. - ppt download

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
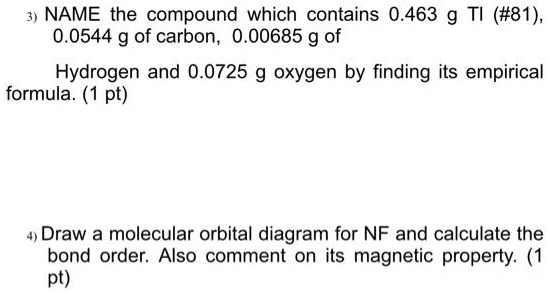
SOLVED: NAME the compound which contains 0.463 g Tl (#81) 0.0544 g of carbon, 0.00685 g of Hydrogen and 0.0725 g oxygen by finding its empirical formula: (1 pt) Draw a molecular
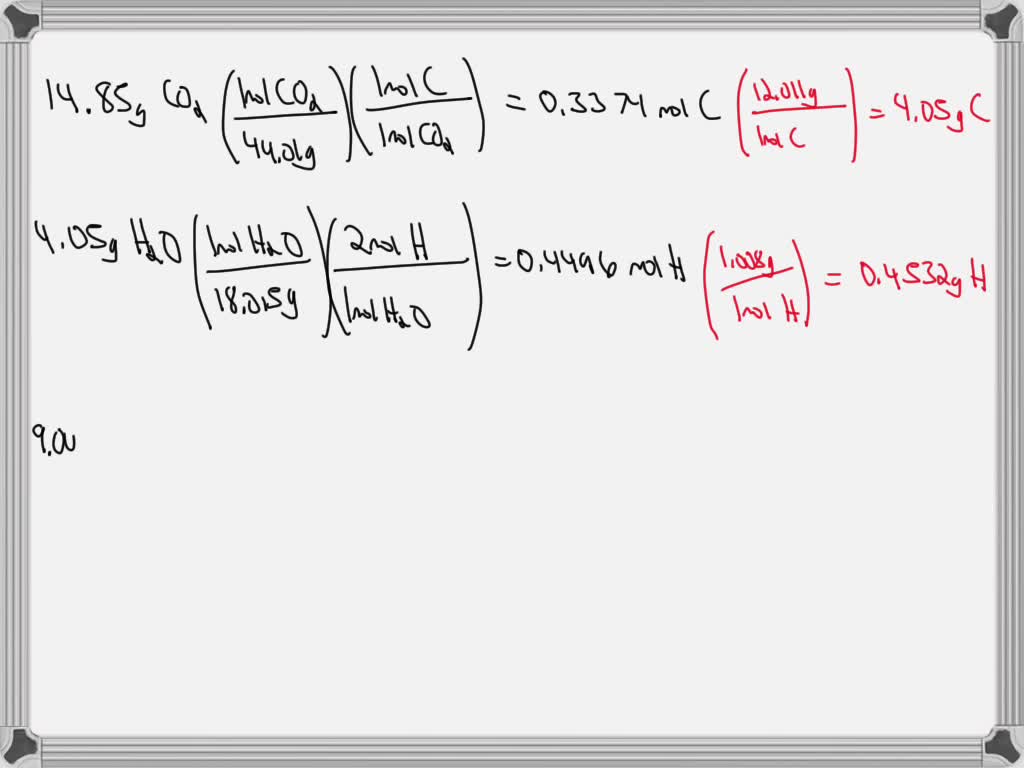
SOLVED: 9.00 g of a certain Compound X, known to be made of carbon, hydrogen, and perhaps oxygen, and to have a molecular molar mass of 152 g/mol, is burned completely in

⏩SOLVED:Find the empirical formula of each of the following…
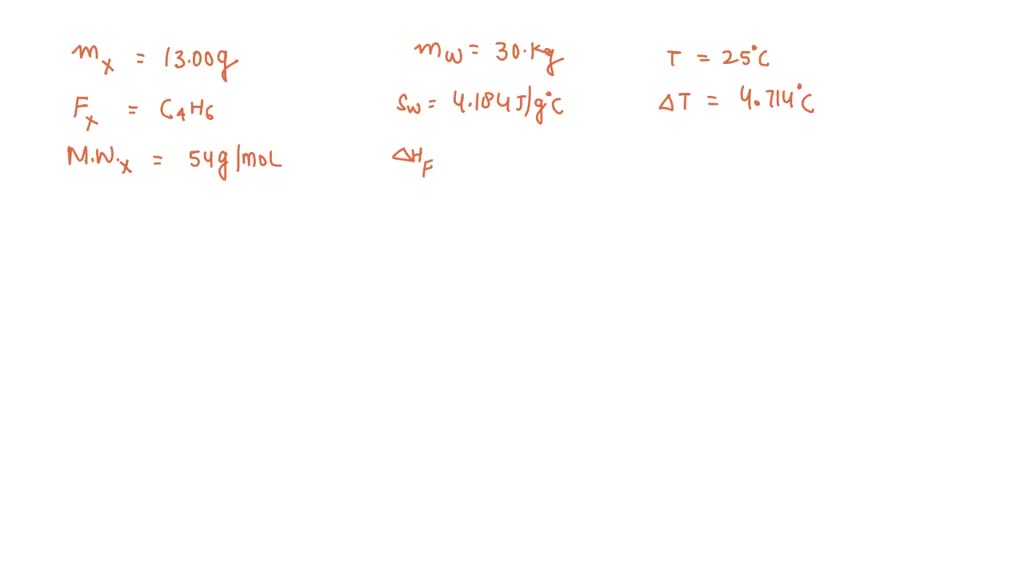
SOLVED: 13.00 g of Compound X with molecular formula C4H6 are burned in constant-pressure calorimeter containing 30.00 kg of water at 25 %C The temperature of the water is observed to rise
A compound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass. What is the empirical formula? - Quora
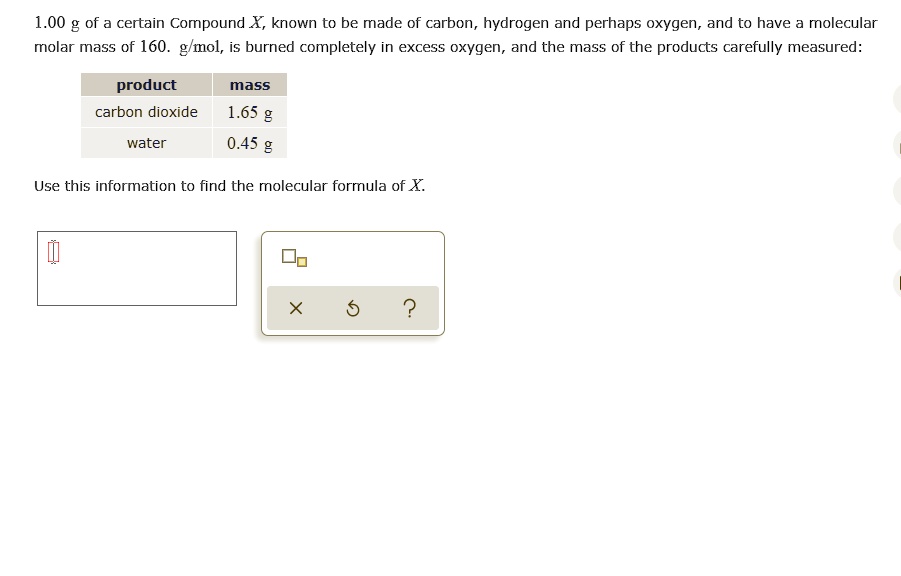
SOLVED: 1.00 g of certain Compound X, known to be made of carbon; hydrogen and perhaps oxygen, and to have molecular molar mass of 160. g mol, is burned completely in excess

Solved The empirical formula of a certain compound is CHN.
Solved] 14.5 grams of sugar (C12H22O11) are dissolved in water to make 1 L

Chem Test 2 Flashcards
de
por adulto (o preço varia de acordo com o tamanho do grupo)