Ethide Laboratories - USP 88 In-Vivo Cytotoxicity Testing
Por um escritor misterioso
Descrição
Learn what USP 88 cytotoxicity tests are available and which ones you will need to meet the regulatory requirements for your medical devices.

Radiolabeled Trastuzumab Solid Lipid Nanoparticles for Breast Cancer Cell: in Vitro and in Vivo Studies
Industry Alignment To Eliminate USP In Vivo Animal Bioreactivity Testing For Polymer Characterization In Pharma Manufacturing

acta 2_2015
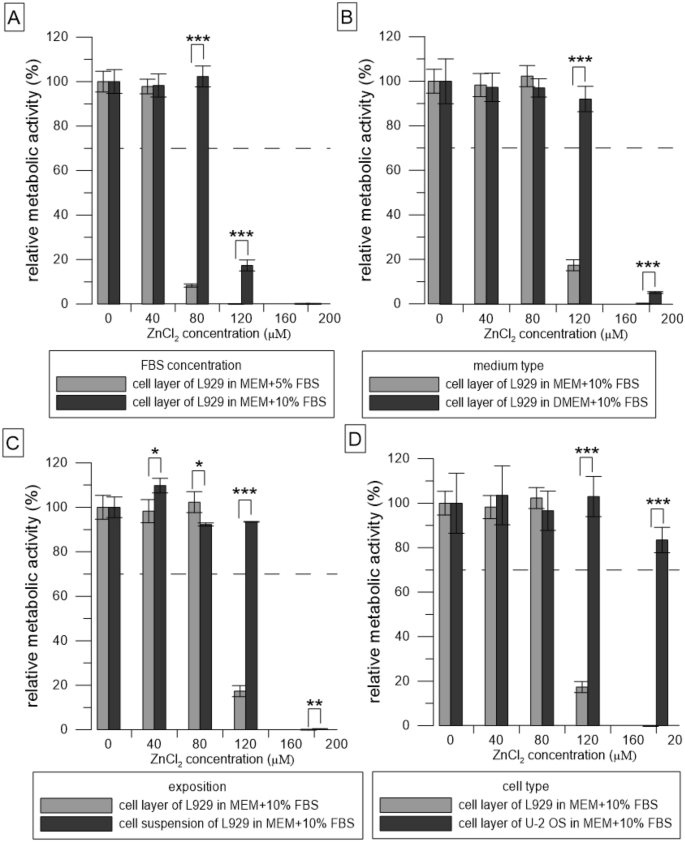
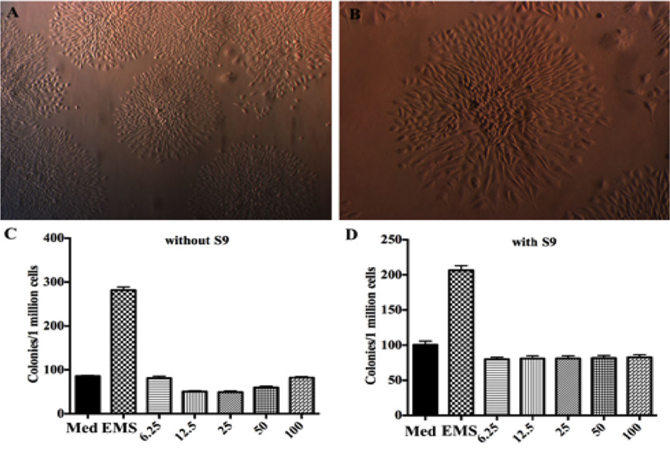
Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
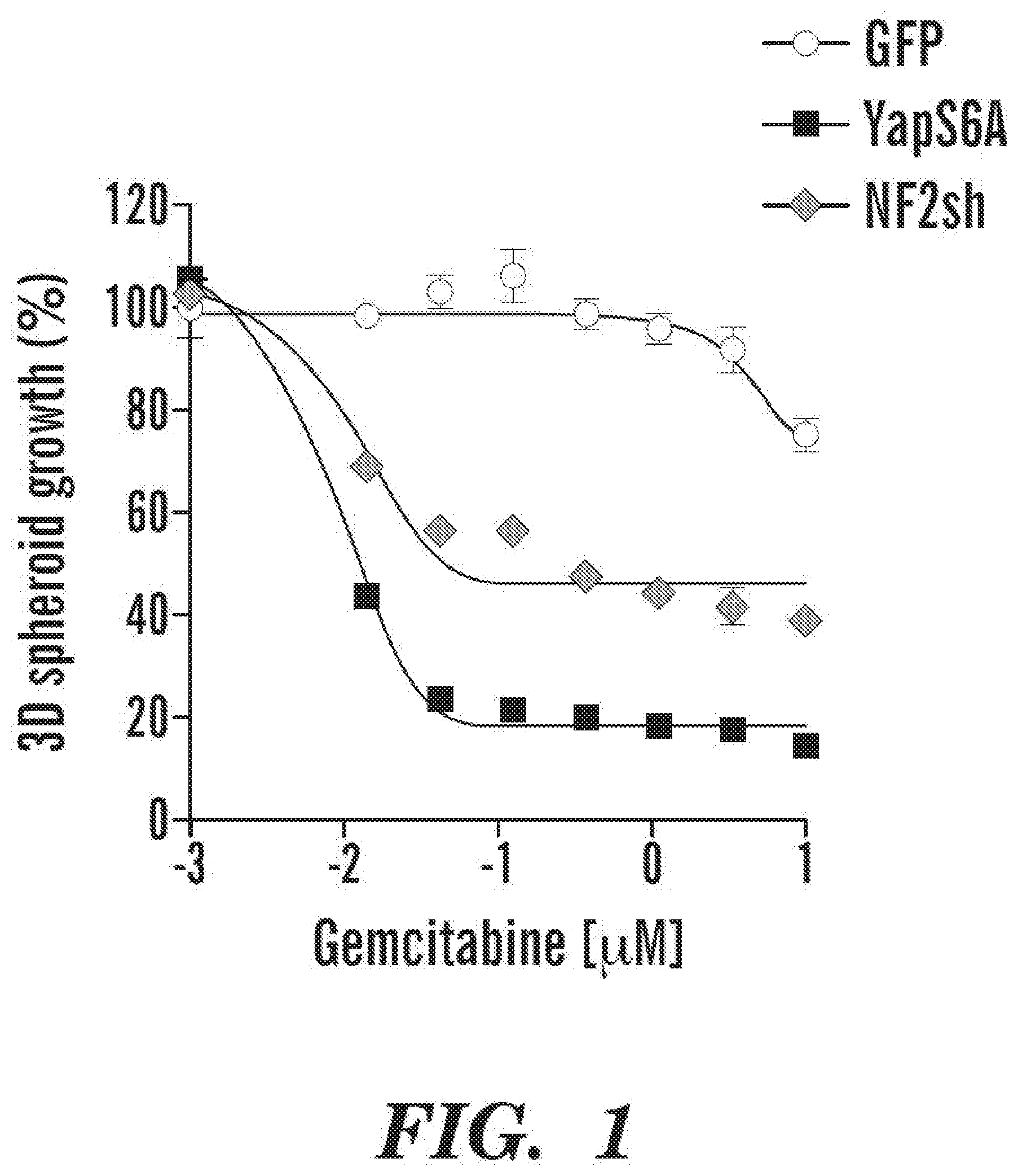
Methods And Compositions Relating To The Diagnosis And Treatment Of Cancer GUJRAL; Taran ; et al. [PRESIDENT AND FELLOWS OF HARVARD COLLEGE]
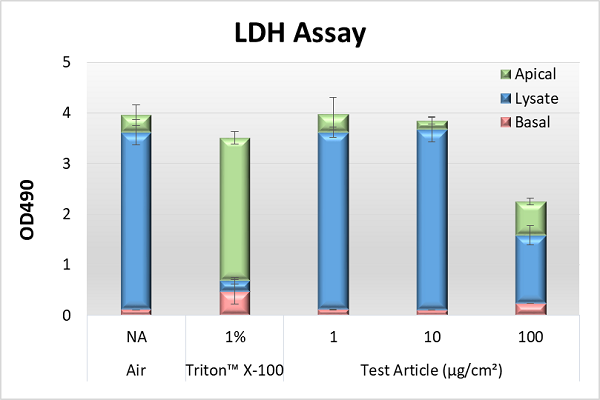
Non Animal Testing, Alternative Test Methods, In Vitro Toxicology, IIVS
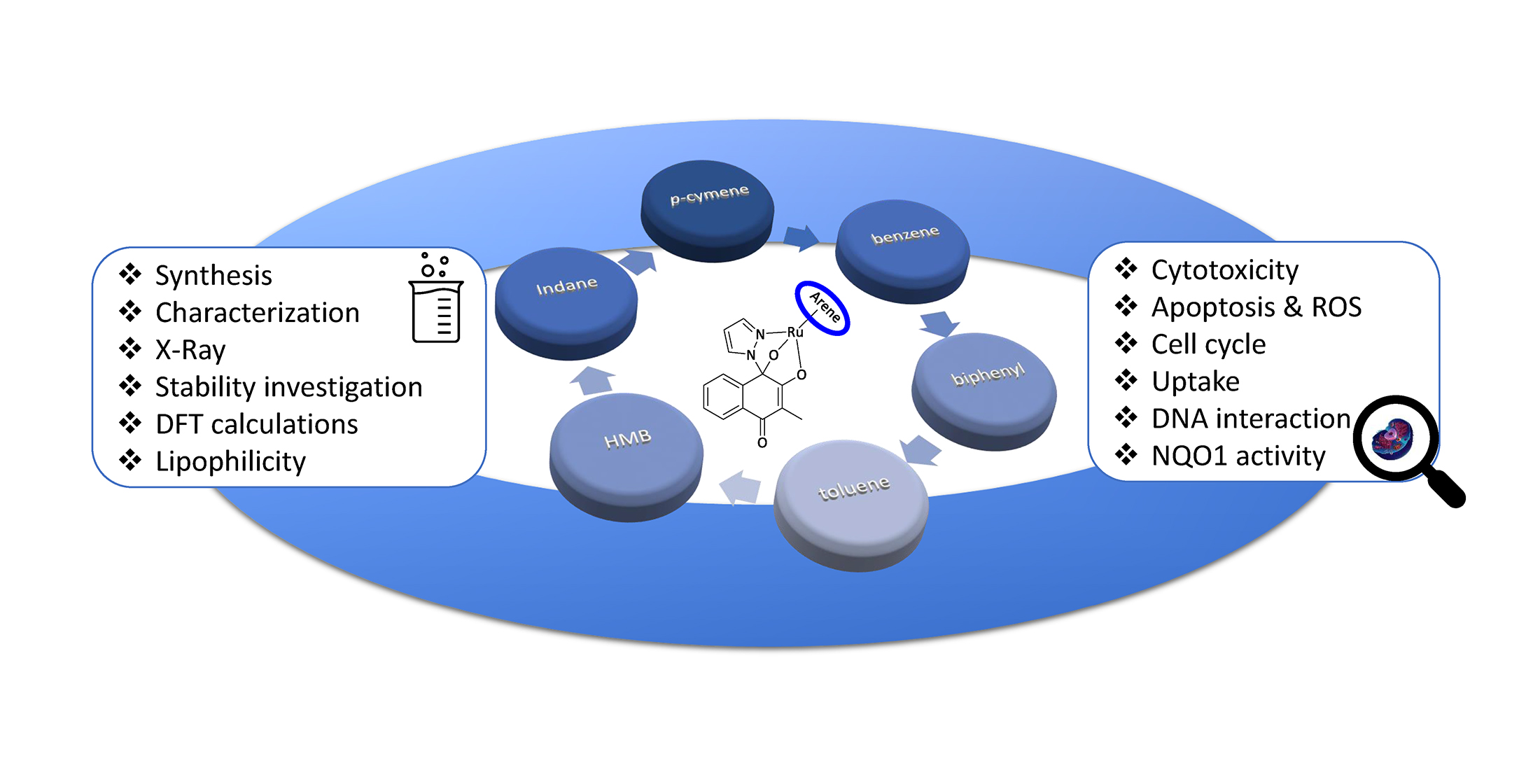
Pharmaceutics, Free Full-Text
In vitro and in vivo biocompatibility of Ti-6Al-4V titanium alloy and UHMWPE polymer for total hip replacement
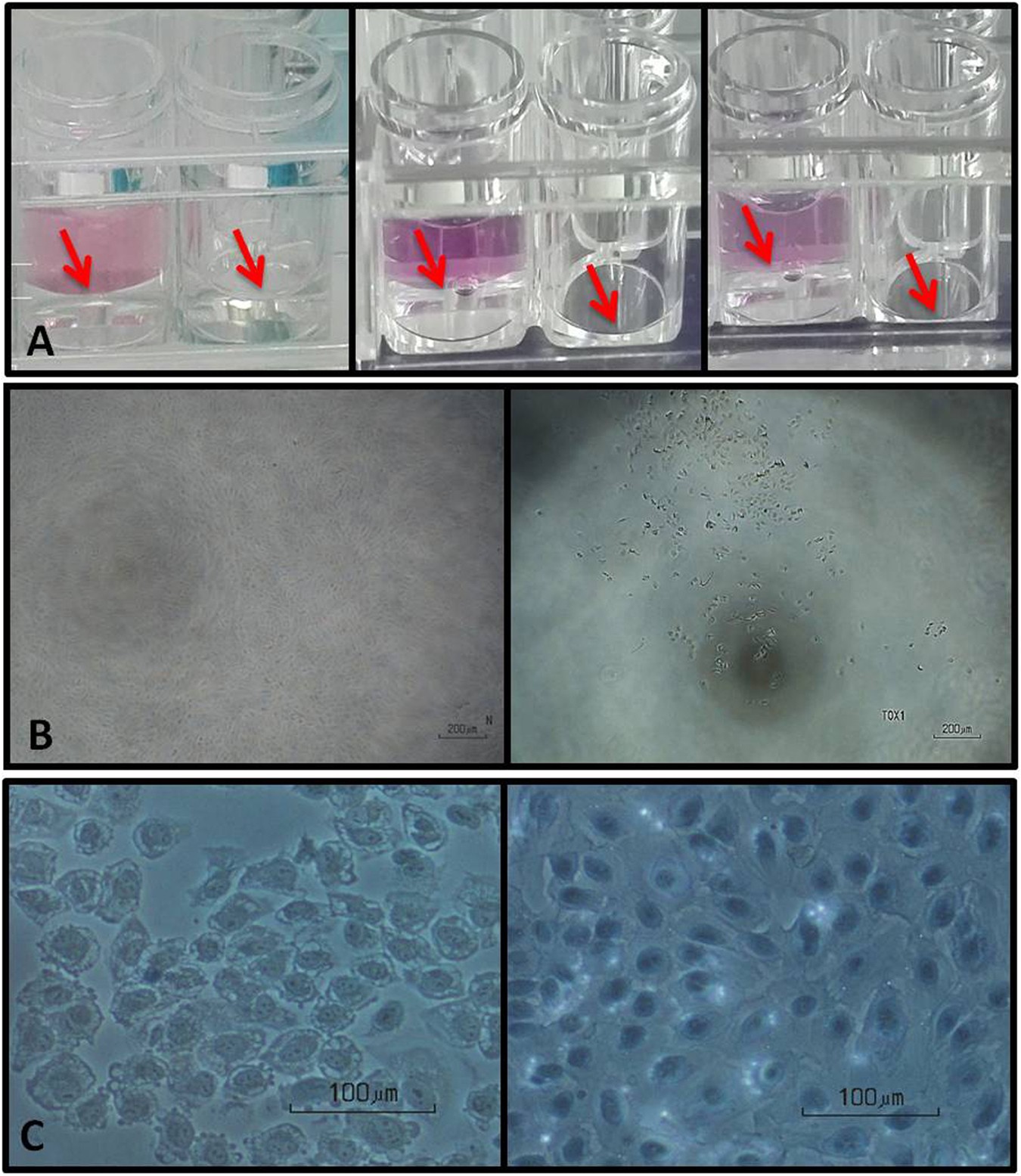
Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation

Cancer Therapy Volume 2 Issue B by Cancer Therapy - Issuu
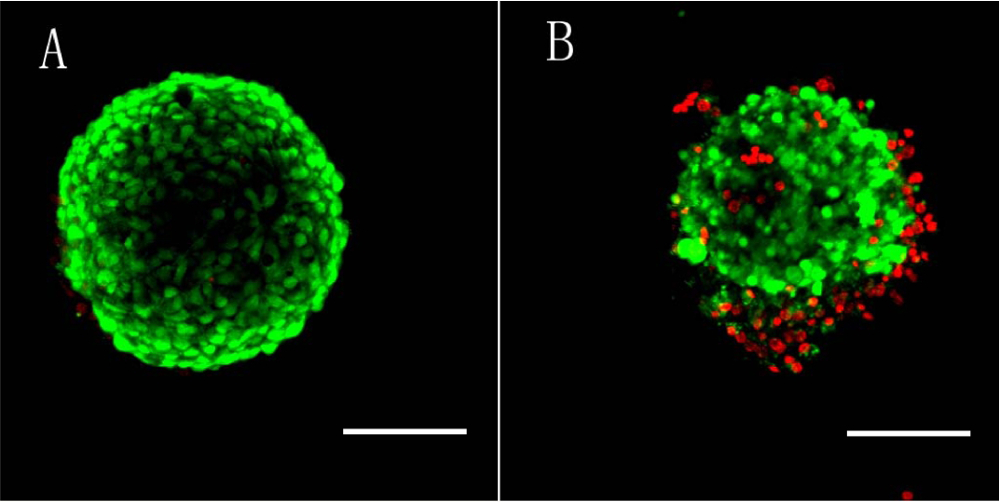
In Vitro Growth of Mouse Preantral Follicles Under Simulated Microgravity
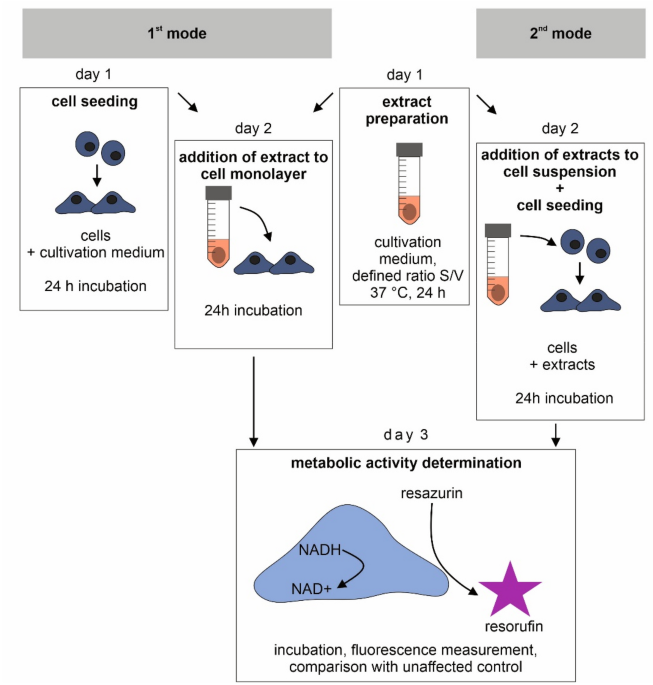
Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
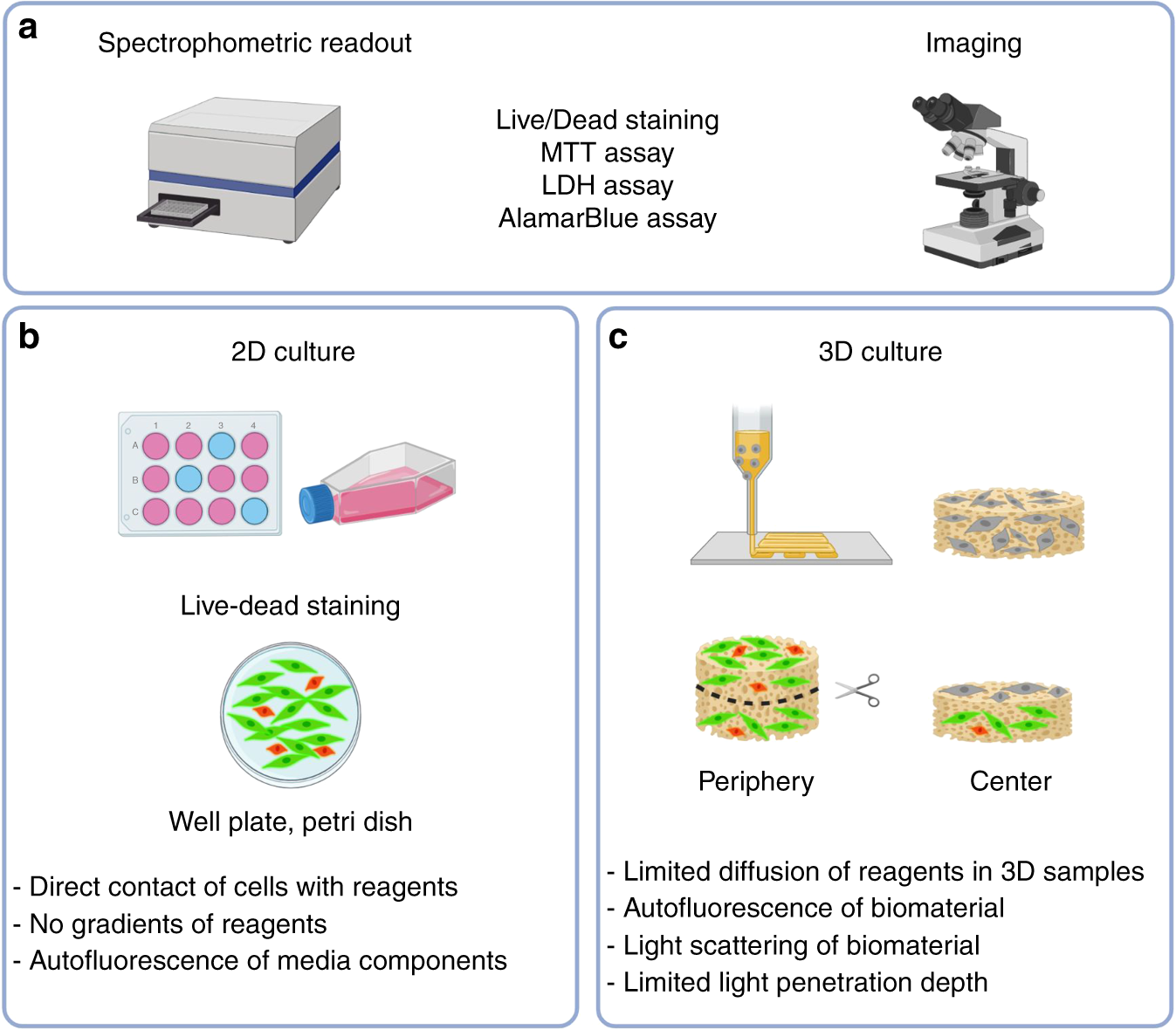
Quality control methods in musculoskeletal tissue engineering: from imaging to biosensors
de
por adulto (o preço varia de acordo com o tamanho do grupo)







