Cetuximab, irinotecan and fluorouracile in fiRst-line treatment of
Por um escritor misterioso
Descrição
Background Combination of chemotherapies (fluoropirimidines, oxaliplatin and irinotecan) with biologic drugs (bevacizumab, panitumumab, cetuximab) have improved clinical responses and survival of metastatic colorectal cancer (mCRC). However, patients’ selection thorough the identification of predictive factors still represent a challange. Cetuximab (Erbitux®), a chimeric monoclonal antibody binding to the Epidermal Growth Factor Receptor (EGFR), belongs to the Immunoglobulins (Ig) grade 1 subclass able to elicite both in vitro and in vivo the Antibody-Dependent Cell-mediated Cytotoxicity (ADCC). ADCC is the cytotoxic killing of antibody-coated target cells by immunologic effectors. The effector cells express a receptor for the Fc portion of these antibodies (FcγR); genetic polymorphisms of FcγR modify the binding affinity with the Fc of IgG1. Interestingly, the high-affinity FcγRIIIa V/V is associated with increased ADCC in vitro and in vivo. Thus, ADCC could partially account for cetuximab activity. Methods/design CIFRA is a single arm, open-label, phase II study assessing the activity of cetuximab in combination with irinotecan and fluorouracile in FcγRIIIa V/V patients with KRAS, NRAS, BRAF wild type mCRC. The study is designed with a two-stage Simon model based on a hypothetical higher response rate (+ 10%) of FcγRIIIa V/V patients as compared to previous trials (about 60%) assuming ADCC as one of the possible mechanisms of cetuximab action. The test power is 95%, the alpha value of the I-type error is 5%. With these assumptions the sample for passing the first stage is 14 patients with > 6 responses and the final sample is 34 patients with > 18 responses to draw positive conclusions. Secondary objectives include toxicity, responses’ duration, progression-free and overall survival. Furthermore, an associated translational study will assess the patients’ cetuximab-mediated ADCC and characterize the tumor microenvironment. Discussion The CIFRA study will determine whether ADCC contributes to cetuximab activity in mCRC patients selected on an innovative immunological screening. Data from the translational study will support results’ interpretation as well as provide new insights in host-tumor interactions and cetuximab activity. Trial registration The CIFRA trial (version 0.0, June 21, 2018) has been registered into the NIH-US National Library of Medicine, ClinicalTrials.gov database with the identifier number ( NCT03874062 ).

S0600/iBET schema: irinotecan and cetuximab with or without

Addition of cetuximab to chemotherapy shortens OS in advanced

Impact of Subsequent Therapies on Outcome of the FIRE-3/AIO

Phase II study of S-1-based sequential combination chemotherapy

Pharmacogenomics of cetuximab in metastatic colorectal carcinoma

ERBITUX (cetuximab) for Head and Neck Cancer and Colorectal Cancer
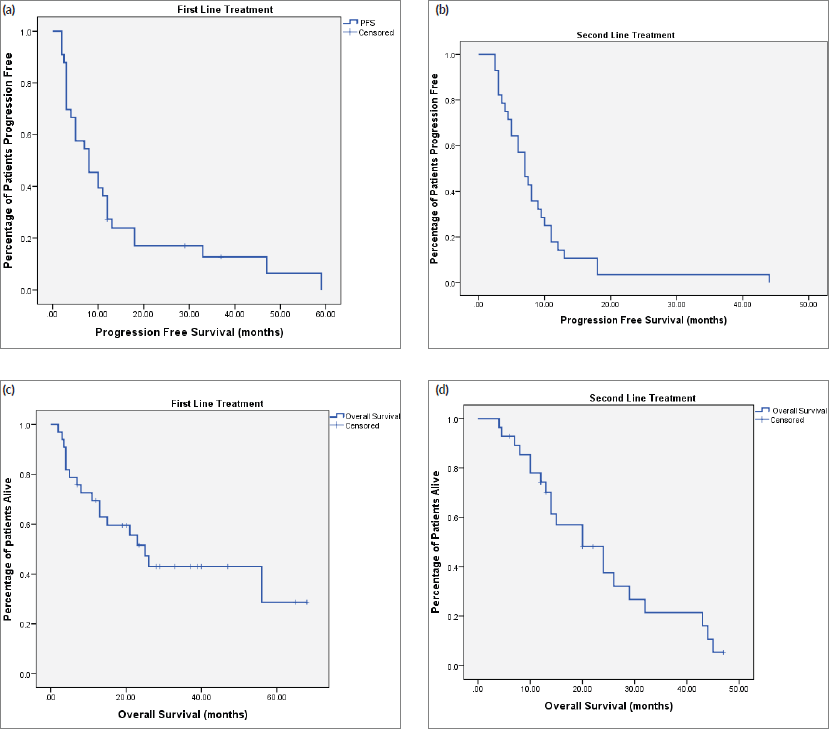
Biweekly cetuximab in combination with capecitabine and

Metastatic Colorectal Cancer, mCRC, HCP

Network Meta-analysis of First-Line Systemic Treatment for
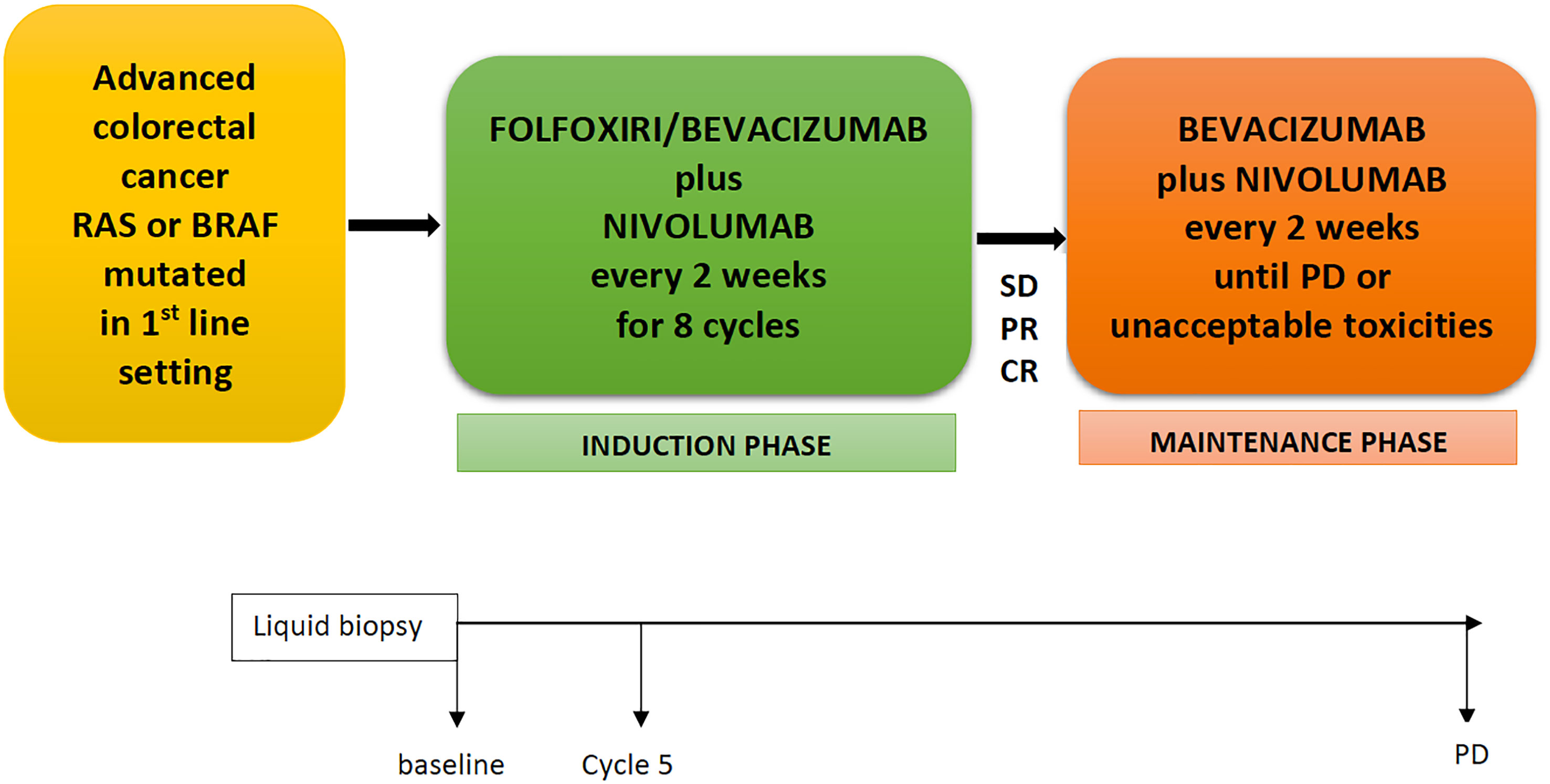
Frontiers FOLFOXIRI/Bevacizumab Plus Nivolumab as First-Line

IJMS, Free Full-Text

Adding Cetuximab to First-Line FOLFIRI Does Not Benefit Metastatic

Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan
de
por adulto (o preço varia de acordo com o tamanho do grupo)






